Moms Heart Starts Beating Again From Baby
Abstract
The vagal activeness of infants is represented by heart charge per unit variability (HRV) and associated with both growth and socioemotional development. The enhancement of an infant's vagal tone activeness might exist benign for evolution. This study explored whether HRV in infants aged 3–eight months tin be enhanced by influencing HRV in mothers (40 dyads). The power of the depression frequency (LF) component of maternal HRV was facilitated using tedious-paced breathing. We investigated whether the modify in maternal HRV afflicted the LF component in infants held by their mothers. In older infants (N = xiv, 6–8 months) the LF ability showed an increment during maternal paced breathing, whereas a delayed increment occurred after termination of maternal paced breathing in younger infants (N = 16, 3–five months). These results prove that the effects of maternal cardiac activity on the infant'south HRV are age-dependent. This age-dependent reactivity of the infant's HRV could be due to the evolution of the inner model in infants which regulates physiological functions, including cardiac activity. This finding might help develop efficient methods for enhancing vagal nerve activeness in infants.
Introduction
Heart rate variability (HRV) is the fluctuation of intervals between heartbeats and has been utilised to estimate vagal nerve activityone, which reflects a core regulation mechanism of the encephalon and trunk. A decrease in HRV is linked to vulnerability to stress whereas an increase in HRV represents physical and mental adaptabilityane. Previous studies reported that HRV in the foetal, neonatal and infant periods could predict diverse aspects of development. The development of HRV in foetuses is related to language skills and psychological development in early babyhoodii. The vagal action of infants, represented by HRV, is associated with both growth and socioemotional development3. These findings led us to infer that the enhancement of an infant'south vagal tone activity might be beneficial for his/her development.
In previous studies, the interactions of heartbeats between mothers and foetuses were observediv,5. The cardiac interaction betwixt mothers and infants was demonstrated past examining the reactivity of HRV in infants to fluctuations in their mothers' respiratory sinus arrhythmia (RSA), which is one of the components of HRV6. In that study, mothers conducted paced breathing while their infants lay on their body with pare-to-skin contact. The RSA in infants during the first 2 months of historic period can exist elevated by increasing their mothers' RSA. However, at iii months of age, the infants' RSA deviated from their mothers' RSA. The authors stated that the reduction of physiological adaptation between 8 and 12 weeks might reflect the shift from an intra-uterine to an extra-uterine status.
This result suggests the phenomenon of cardiac synchronisation betwixt mother and baby might be dependent on the infants' age. In neonatal studies, the respiratory command of premature infants has been influenced by the caregivers' cardiac rhythms during pare-to-skin contact7. Such skin-to-peel contact has a favourable effect on parasympathetic nerve activities in premature infants and mothersviii. Furthermore, in 3-month old infants, vocal synchrony betwixt female parent and infant during confront-to-face interactions coordinates their heart rhythms9.
However, developmental changes in the physiological interaction of cardiac activity in mother and babe later on iii months take not been clarified. During the period of rapid development of motor functions that enable itch and walking, specifically at 3–8 months former, the prominent development of the autonomic nerve supports such motor functions, reflected by an increase in infant HRV, which is parallelly observed10,11. Because this finding, the characteristics of cardiac interactions betwixt mother and baby might change during such a period.
We explored whether the HRV in infants at 3–8 months erstwhile, during the rapid development of their motor functions and the autonomic nerve control, is influenced by HRV in mothers as reported past Van Puyyelde6, subsequently the intra-uterine effect disappears. To attain this aim, we manipulated mothers' respiration to increment their HRV, in a situation where they kept concrete contact with their infants. It was reported that breathing at a charge per unit of approximately six cycles per minute (cpm) increased the low frequency (LF) component ability of HRV12,13. HRV is influenced by two chief physiologic fluctuations: RSA and the Mayer waves in blood pressure. RSA is a naturally occurring variation in the heart charge per unit that occurs during a breathing cycle, where the eye rate increases during inspiration and decreases during expiration. This fluctuation usually occurs at a frequency of three–4 seconds. The respiration rate of a healthy developed woman is in the range of 14–18 breaths per minutefourteen,15, making natural RSA occurring in the frequency range of three–iv seconds. Mayer waves in claret pressure occur at a frequency of 0.1 Hz or a 10-second periodicity. With a animate rate of vi cpm or a 10-2nd periodicity, information technology is thought that RSA and the Mayer wave become synchronised and tin can enhance HRV due to their resonance16.
We predicted that the HRV of infants hugged by their mothers would be enhanced by increasing the mothers' HRV through paced respiration. The influence of the mothers' HRV on the HRV in infants might exist mediated by the physical synchronisation of cardiac activities between mother and babe, and the magnitude of the influence might depend on the baby's age. Specifically, we explored whether the influence of the mothers' HRV on the infants' HRV was different betwixt relatively older infants (6–8 months old) and younger infants (3–5 months sometime). Furthermore, the directions of HRV influences between female parent and infant were estimated past calculating transfer entropy (TE) of heartbeats between mothers and infants17,18.
Results
Because the wide individual differences in the LF, the high frequency (HF) components of HRV in mothers and infants and the TE between mother and infant heartbeats at each baseline, we conducted a 3-mode repeated measures analyses of variance (ANOVA) (developmental stage (older vs. younger) × condition (natural- breathing vs. paced- breathing) × period (pre-rest, respiration 0–v min, respiration five–10 min, respiration ten–xv min and mail service-rest) to assess the changes in those indices from the baselines. Although we mainly focused on the LF component of HRV to mensurate the effects of paced respiration on HRV12, we also examined the HF ability and LF/HF ratio of HRV.
The interaction between the condition and period was pregnant for the LF power in mothers, (F (4, 84) = 9.8639, p < 0.001, partial η two = 0.320; Fig. 1(a)). In the paced-breathing condition, the LF ability during the periods of 0–5 min, 5–x min and 10–fifteen min was significantly higher than at the pre-rest catamenia (p < 0.001, p = 0.002 and p = 0.001, respectively) as well as during the post-residual period (p < 0.001, p = 0.005 and p = 0.002, respectively). During the periods of 0–5 min, five–10 min and 10–fifteen min, the LF ability in the paced-animate status was significantly higher than in natural-breathing condition (p < 0.001, p = 0.001 and p = 0.002, respectively). The HF power in mothers showed neither significant master effects nor interactions (Fig. ii(a)).
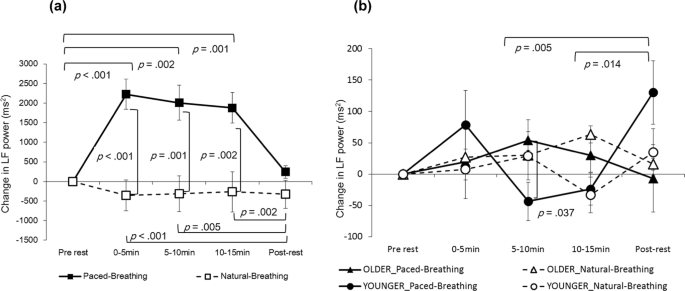
Modify in LF power of HRV. Left (a) in mothers (north = 22), right (b) in infants (n = xxx). Error bars indicate standard errors.
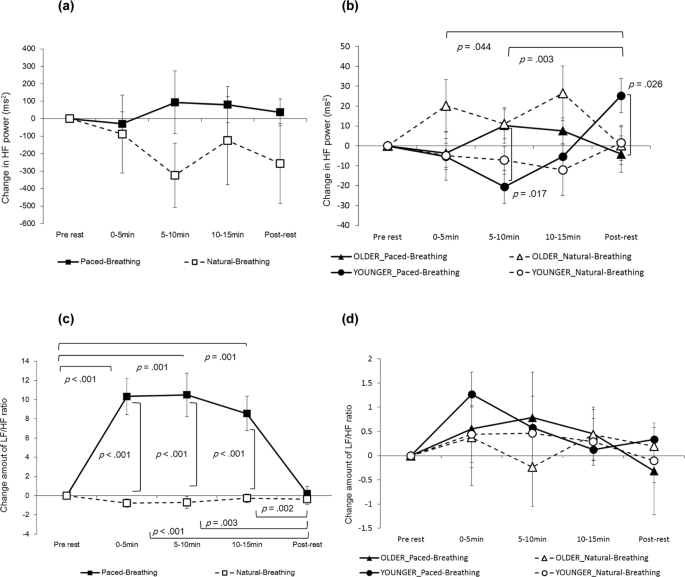
Change in HF ability of HRV and change corporeality of LF/HF ratio. Left (a) in mothers' HF power (n = 22), right (b) in infants' HF power (n = 30). Left (c) in mothers' LF/HF ratio (north = 22), correct (d) in infants' LF/HF ratio (n = thirty). Error confined indicate standard errors.
In relation to the change in the LF/HF ratio in mothers, the interaction between status and catamenia was significant (F (2.655, 55.755) = 14.085, p < 0.001, partial η 2 = 0.401; Fig. two(c)). In the paced-breathing status, the LF/HF ratio for power in periods of 0–5 min, 5–10 min and 10–15 min was significantly higher than in the pre-rest menses (p < 0.001, p = 0.001 and p = 0.001, respectively) as well as in the post-balance menses (p < 0.001, p = 0.003 and p < 0.002, respectively). In periods of 0–five min, 5–x min and 10–15 min, the LF/HF ratio in the paced-breathing condition was significantly higher than in the natural-animate status (p < 0.001 in all atmospheric condition).
The LF power in infants indicated that the interaction between status, menstruum and developmental stage was pregnant (F (4, 112) = 2.757, p = 0.031, partial η 2 = 0.090; Fig. 1(b)). We examined a elementary interaction upshot between catamenia and developmental phase for each condition. In natural-breathing atmospheric condition, the simple interaction was not significant (F (4,112) = 1.477, p = 0.214, partial η 2 = 0.050). On the other hand, in paced-animate weather, the simple interaction was significant (F (iv,112) = four.586, p = 0.002, fractional η ii = 0.141), suggesting that the LF power of the younger infants was significantly lower than that of the older infants during the five–10 min period (p = 0.037). Additionally, in younger infants simply, the LF power during the post-remainder period was significantly higher than during the periods of v–10 min and 10–15 min (p = 0.005 and p = 0.014, respectively). The simple interaction effect between period and condition was non significant at either developmental stage.
The HF power in infants indicated that the interaction between condition, period and developmental stage was close to pregnant (F (4,112) = 2.369, p = 0.057, partial η 2 = 0.078; Fig. 2(b)). In natural-breathing weather condition, the simple interaction was close to significant (F (4, 112) = ii.435, p = 0.051, partial η 2 = 0.080). In paced-breathing conditions, the simple interaction was meaning (F (4,112) = half-dozen.949, p < 0.001, partial η two = 0.199), suggesting that HF power in older infants was significantly higher than that in younger infants during the 5–10 min flow (p = 0.017). The younger infants' HF ability was significantly higher than the older infants during the post- balance menstruation (p = 0.026). In younger infants merely, the HF power during the mail service-rest period was significantly college than during the 5–10 min and x–15 min periods (p = 0.044 and p = 0.003, respectively). The simple interaction outcome between menstruum and condition was not significant at either developmental stage. The modify in infants' LF/HF ratios demonstrated no meaning main furnishings or interactions (Fig. 2(d)).
The interaction between status and period was significant for the logged values of TE from mothers to infants (F (4, 68) = iii.103, p = 0.021, fractional η 2 = 0.154; Fig. 3), suggesting that the logged values of TE in paced-animate conditions was significantly lower than during natural-breathing conditions during the mail service-residue period (p = 0.018). No other effect was significant. A repeated measure ANOVA measuring changes in the logged values of TE from infants to mothers showed no pregnant main effects or interactions.
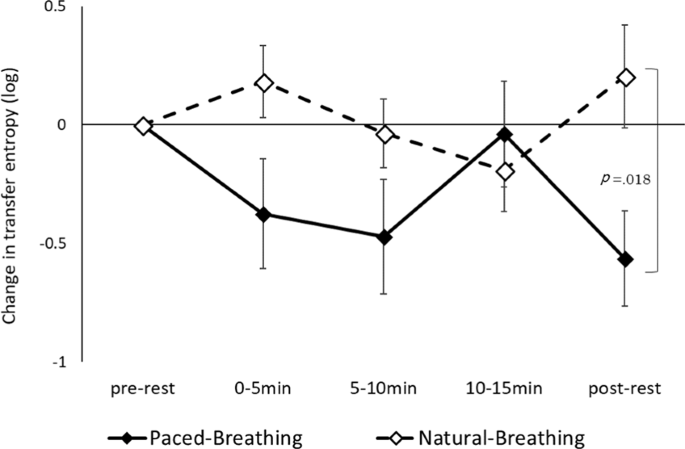
Alter in transfer entropy from mothers to infants (xix dyads). Error bars signal standard errors.
A Pearson'due south correlation analyses of the mothers' LF power, the infants' LF power and the infants' HF power were performed to explore the associations betwixt mothers' and infants' cardiac activities in the paced-breathing conditions (Table ane). The LF ability of mothers during the respiration 0–5 min period and ten–15 min period was strongly related to the LF ability of infants during the respiration 10–15 min period for the older group (r = 0.863, p = 0.006 and r = 0.779, p = 0.023). At that place was no correlation between the LF power of mothers and the HF power of infants. For older infants, the LF power during the respiration 0–5 min catamenia was strongly related to the HF power during the respiration 0–5 min period (r = 0.715, p = 0.046). For younger infants, the LF ability during the 5–10 min period of respiration was strongly related to the HF power of post-rest (r = 0.691, p = 0.018).
On the basis of the correlational analyses, a regression analysis was performed using the mothers' LF changes and developmental stage as independent variables and the infants' LF changes as a dependent variable. The multiple linear regressions were calculated to predict the modify in infants' LF power in relation to a change in mothers' LF power and the developmental stage during each menstruum of the experiment. The best plumbing equipment model for predicting the alter in infants' LF power during the respiration 5–10 min period was a linear combination of the LF power of mothers during the respiration 0–5 min period and developmental phase (F (2, 16) = four.521, p = 0.028, with an R 2 of 0.361; Table ii, Fig. 4a). Both the LF ability of mothers and developmental stage had meaning furnishings (B = 0.039, SE = 0.017, p = 0.032 and B = 129.352, SE = 57.107, p = 0.038). The addition of the interaction variable did not significantly improve prediction (ΔR 2 = 0.016, ΔF = 0.393, p = 0.540).
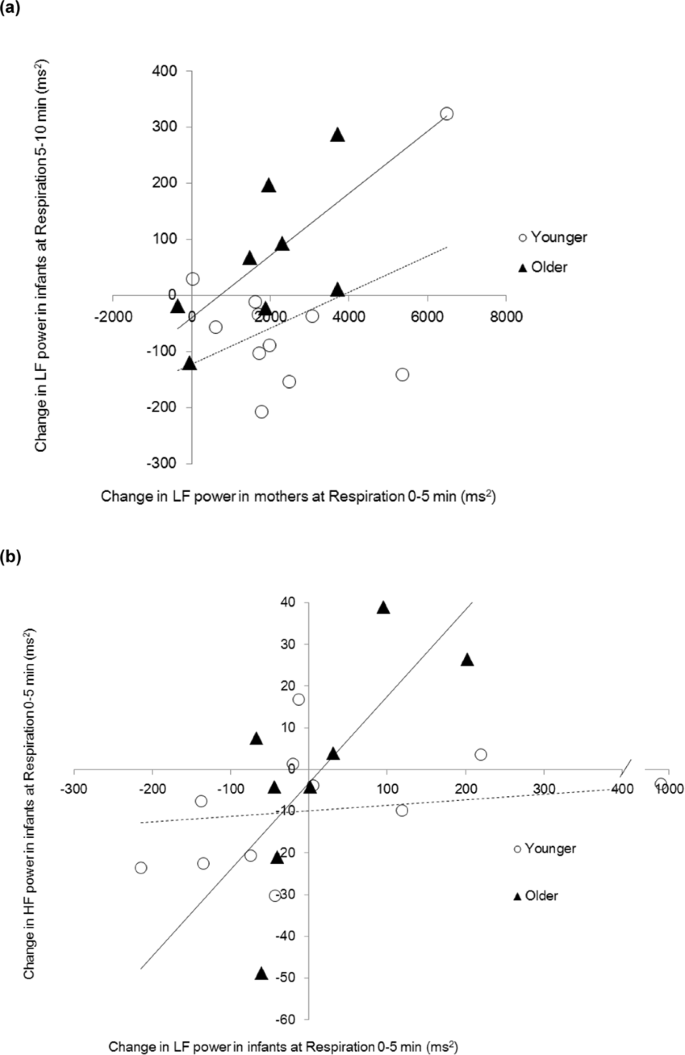
(a) Clan betwixt the alter in LF ability of infants and the change in LF power of mothers (19 dyads). (b) Association between the alter in HF ability and the change in LF ability of infants. (due north = 19) Solid line; regression line for older infants, cleaved line; regression line for younger infants.
Significant associations between mothers' LF power and infants' HF ability were not found in either the older or younger groups.
A similar regression analysis was performed to track associations between infants' LF alter and HF change during each developmental stage. Developmental stage significantly chastened the association between the alter in LF power of infants during the respiration 0–5 min period and the change in HF power during the respiration 0–five min menstruation (ΔR 2 = 0.291, ΔF = vii.595, p = 0.015; Table three). A elementary slope exam demonstrated that LF power was associated positively with HF power in the older group (B = 0.194, SE = 0.064, p = 0.008, Fig. 4b), while no significant association was observed in the younger group (B = −0.003, SE = 0.019, p = 0.856).
Word
The maximum heart rate oscillations at respiratory frequency occurred at approximately 0.i Hz (6 bpm)16. The respiration-induced changes in HRV may function as a positive feedback loop, spiralling farther increases in HRV due to feedback from the centre to the key nervous system through the vagal afferent system. In this study, a robust increase in the ability of the LF component and the LF/HF ratio of HRV, as well every bit no alter in the HF power of HRV, in mothers performing paced breathing were observed. These results are consequent with previous findings19,20. These findings verified the validity of the experimental manipulation performed in this study.
The influence of mothers' enhanced HRV while performing paced-breathing on their infants' HRV depended on the infant'south age; the increase in LF power in older infants was greater than in younger infants. The multiple regression analysis indicated that the LF power of infants increased according to an increase in the mothers' LF ability, and this tendency was greater in older infants than in younger infants. The TE assay suggested that the association of HRV between mothers and older infants was due to the trounce past beat influence of the mothers' cardiac activity on the infants' cardiac activity. The results indicating that the influences of the infants' cardiac activity on the mothers' cardiac action were relatively small seems rational, considering that infants have much smaller hearts than their mothers, thus their physical touch on should exist smaller. The middle charge per unit of an adult can be easily synchronised to an external sequential pulse such as sound and low-cal, fifty-fifty when the stimulus is weak, via concrete entrainment21. Our finding suggests that such mechanisms should be developed in infants at to the lowest degree by 8 months old.
Interestingly, while the increment in older infants' LF power due to the influence of mothers' LF power quickly returned to the baseline just afterward the termination of paced breathing, the LF power in younger infants showed delayed increase during the postal service-remainder menses when mothers were no longer performing paced breathing. Also, the correlation analysis institute no correlation between the mothers' LF power and the younger infants' LF power during the paced breathing menstruum, whereas a tight correlation of mother-baby LF power was observed in older infants during this period. These results led us to infer that the effects of the mothers' HRV on the infants' HRV are not a merely passive phenomenon but rather an active miracle mediated by some internal mechanisms in infants. In other words, some computational processes in the infants' brain and body might mediate the observed increase in the infants' HRV via the influence of the mothers' HRV. Probably, such mechanisms in younger infants are young and thus accept a longer time to process the mothers' cardiac signals, resulting in the delayed increase in the infants' HRV.
A possible explanation for this type of machinery can exist plant in the predictive coding model22 proposed by cognitive neuroscience. From this perspective, the brain does non just react to sensory input passively, only it constitutes an inner model to predict a future state. Perception and behaviour are actively synthetic by comparison the input sense signal with the inner model prediction, then calculating their gap (predictive mistake) and regulating to minimise the prediction error. Recently, the principle of predictive coding has been expanded into the perception of the inner trunk, including heartbeat, which is called interoception23. Signals from the mother's cardiac action might reach the babe's encephalon via tactile and auditory sensory modalities and might distract their inner model from the heartbeat resulting in a prediction error. In older infants with a relatively matured inner model, the beat by beat regulation of the heartbeat might be conducted to minimise the prediction error, resulting in an increment in their HRV. When the signals of the mother's cardiac action are terminated, contrary regulation of the inner model tin can quickly render the infant's HRV to the baseline level. In younger infants, the inner model function should be premature, and the regulation of their cardiac action based on the prediction fault is delayed. Thus, the effects of mothers' cardiac activeness on infants' HRV might announced after a necessary adding to reduce the prediction error has been performed.
The mother's paced animate caused increases in HRV simply in the LF power, not in the HF power. Previous studies have reported that vagal nerve action is the master correspondent to the HF component24,25 and LF component is non affected past respiration26,27, and so this seems to be a specific effect of paced breathing. On the other mitt, a positive correlation was found between infants' LF power and infants' HF power. In add-on, regression analysis indicated an increase in infants' HF ability, equally infants' LF power was significant in the older group merely, not in the younger group. This result implies that the amplification of mothers' LF ability due to paced breathing affected infants' LF power through physical phenomena, and an increase in infants' LF power caused an increment in the infants' HF power due to inner mechanisms in infants in the older group only. Because the HF ability of infants' HRV reflects RSA, governed past efferent vagal nerve activity, this finding suggests that there is an influence of mothers' HRV on infants' HRV through a peripheral route and also via the efferent regulation of cardiac activity. This reasoning is consistent with the above-described interpretation from the predictive coding perspective. Among the possible routes of the influences of mothers' HRV on infants' HRV, the furnishings of mothers' respiration should be considered forth with those of mothers' cardiac action above described. It would be hard to conclude that infants' breathing itself changes due to their mother'south paced breathing, however it is possible that the mothers' chest movements affect the infants' HRV. Unfortunately, we were unable to accurately mensurate the respiration of the mothers and infants, so this question was not accountable from the present study and remains for the future.
Some limitations in this study should be recognised. Firstly, the sample size was minor, although it corresponded to a previous study5. Thus, the findings obtained in this study should be verified and generalised using a larger sample size and a wider age range in infants. Secondly, because this study was exploratory and observational, the underlying mechanisms of the effects of mothers' cardiac activity on infants' cardiac action requires further enquiry. Additionally, this study did not measure the respiration of the mothers and infants, so, it was not possible to provide a detailed examination of the dynamics of cardiac and respiratory activities betwixt mothers and infants. Finally, the long-lasting benign effects of the enhancement of infants' HRV on mental and physical development are critical, but across the scope of this study. Such effects should exist examined using longitudinal designs in the hereafter.
In spite of such limitations, to the best of our cognition, this study is the first to demonstrate that HRV in infants can exist enhanced by their mothers' HRV via direct skin-to-skin contact and that this outcome is age-dependent; the reactivity of infants' HRV depends on their developmental phase. In addition, this historic period-dependent reactivity of infants' HRV could be due to the development of infants' inner model which regulates physiological functions including cardiac action. The present findings might be useful for developing efficient methods to enhance vagal nervus action in infants, which would be beneficial for infants' well-being.
Method
Participants
Forty dyads of healthy mothers and infants participated in this written report. This sample size was determined on the basis of a related previous study5 (N = 11). The dyads were recruited according to the historic period of the infants in months, via the database of Unicharm Co. which is based in Kagawa prefecture, Japan. The infants' ages ranged from three to viii months (hateful age = 5.43 months, SD = 1.26 months). In that location were eighteen males and 22 females, with a weight range of v.three–eight.9 kg (mean weight = 7.35 kg, SD = 0.91 kg). The infants were categorised into an older group (vi–8 months sometime; Due north = 19; 12 females) and a younger group (3–5 months erstwhile; N = 21; 10 females). The female parent's age was 25–41 years old (mean age = 32.63 years, SD = 4.32). Due to infants' states (e.chiliad. crying) and technical failures of recoding HR, 19 dyads with complete data (older group, North = 8; younger group, Northward = 11) were finally subjected to statistical analyses to examine mother-infant cardiac interactions. The bones attributes of the participants are provided in Table 4. We conducted this study in compliance with the Announcement of Helsinki, and all experimental protocols were approved by the Ethics Committee of Nagoya University. Written informed consent was obtained from all parents of infants before the first experimental session. Clinical trial registration details are as follows: Clinical Trial Registry Number UMIN000034772 five/11/2018 [University Hospital Medical Information Network], retrospectively registered.
Written report design
This study examined the influence of mothers' enhanced HRV through the manipulation of their respiration on the HRV in their infants. A crossover pattern was adopted where all the dyads participated in both a paced-breathing session and a natural-breathing session on separate days (range of intervals; 1–xx days). The order of the breathing conditions was counter-counterbalanced between dyads.
Measurement of heart rate and betoken processing
Small heart rate sensors (My Crush, Matrimony TOOL Co., Japan, xiii g, twoscore.8 mm × 37.0 mm × 8.9 mm) were attached on the chests of mothers and infants to tape their heartbeats (R waves of electrocardiogram). Every bit this sensor was article of clothing and did not have whatsoever electrical cables, information technology did not disturb the infant'south movements. The LF (mothers' LF, 0.04–0.xv Hz; infants' LF, 0.04–0.24 Hz) power and the HF (mothers' HF, 0.15–0.iv Hz; infants' HF, 0.24–1.04 Hz) power of each five-minute catamenia (pre-rest, respiration 0–5 min, respiration 5–10 min, respiration ten–fifteen min and post-residuum) were computed using the Fast Fourier Transform. The frequency bands of the LF and HF ability of infants were determined according to contempo recommendations promulgated for HRV in infants28. These indexes were calculated using the specialised software (RRI Analyzer 2, UNION TOOL Co., Japan) for the heart rate sensor used in this report.
Manipulation of respiration
During the paced-breathing session, mothers were instructed to breathe at a footstep of six cycles per minute (cpm), to reach a 4-second inspiratory menstruum and 6-2d expiratory flow at each cycle, to raise the LF power of HRV. The breathing pace was guided past a visual pacer on a reckoner monitor. During the natural-animate session, mothers were instructed to breathe at their natural pace, every bit usual.
Procedure
The experimental sessions were conducted at an experimental chamber where temperature and humidity were kept consistent (24 °C, 55%). Later an examiner explained the object and methods of the experimental sessions and received consent, the mothers answered a questionnaire to collect information on their infants' weight and developmental milestones. Prior to the start of the experiment, a heart rate sensor was fastened to each mother and infant. Every baby's diaper was changed to a new diaper earlier initiation of the experimental process, to exclude the influence of any discomfort.
The participants were allowed to remainder for 5 minutes while the infants were laid on a mattress and mothers sat on the floor. After the five minutes pre-rest menstruation, mothers held their infants in their artillery and sat on a chair. The mothers were instructed to exhale at a step of 6 cpm (paced-breathing session) or at a natural pace (natural-breathing session) for fifteen minutes. While the mothers were holding their infants in their arms, their bodies were in full contact throughout the manipulation of respiration to induce common concrete influences. After this respiration period, participants were instructed to remainder for 5 minutes over again (post-residuum period) in the same way as the pre-residue period.
The behaviour of mothers and infants were recorded by a video camera to check for whatever problems during the experimental session. Afterwards each experimental session, mothers were asked to reply a questionnaire by post about their infant'due south profile and health condition at birth. According to the results of the questionnaire, the participants were healthy mothers and infants without any abnormalities at nascency and were non receiving handling for disease.
Transfer entropy
TE is a non-parametric statistic, measuring the direct transfer of information between 2 fourth dimension-series processes, calculated using the following formula. In other words, TE reflects the magnitude of one fourth dimension-serial process' influence on the other.
$${T}_{Y\to X}=\sum _{{10}_{t+one},{x}_{t}\in Ten}\sum _{{y}_{t}\in Y}p({x}_{t+ane},{10}_{t},{y}_{t})\log \,\frac{p({x}_{t+1}|{x}_{t},{y}_{t})}{p({ten}_{t+1}|{ten}_{t})}$$
where Y and X are probabilistic functions and y t and x t are values of the component of Y and X at a time indicate t. In this report, the TE betwixt the heartbeats of mother and infant was calculated using the post-obit formula, for two directions: from female parent to baby and infant to mother. When calculating TE, the stock-still bin approach was used to estimate the probabilities in the in a higher place formula, by allocating the data points to bins of 200 msec. Thus, y t and x t , mean occurrence/non-occurrence of a peal of R wave, indicated 1 or 0. TE was calculated at each 5-minute period (pre-residue, respiration 0–five min, respiration 5–10 min, respiration 10–15 min and post-residuum) during the experimental procedure. As the TE scores were sometimes small, the logged TE values were subjected to statistical analyses.
Statistical assay
Repeated measures ANOVAs with a between-participant factor of developmental stage (older, younger) and 2 within-participant factors of condition (natural-breathing, paced-breathing) and periods (pre-balance, respiration 0–5 min, respiration 5–ten min, respiration 10–15 min and mail-rest), were performed to analyse the LF and HF powers of HRV in mothers and infants, separately. To obtain the logged values of heartbeat TE, the same repeated measures ANOVAs were performed, to assess the management of influences from mother to infant and from babe to mother, separately. The Greenhouse-Geisser correction was applied when a violation of sphericity was observed. Post-hoc analyses were performed by Bonferroni tests (p < 0.05). Partial eta-squared was computed every bit the outcome size.
A series of regression analyses was conducted to examine associations between the mothers' HRV and the infants' HRV in the younger and older groups. We firstly performed exploratory correlation analyses between the LF power of mothers, the LF power of infants and the HF ability of infants. With reference to results of the correlation analyses, we chose changes in mothers' LF power during the 0–v min, five–10 min and ten–15 min periods as possible independent variables and the modify in infants' LF ability during the 0–5 min, 5–10 min, ten–15 min and postal service-residuum periods every bit possible dependent variables to perform a regression analysis. We also conducted a regression analysis to examine associations between the infants' HF ability and mothers' LF power. Furthermore, we conducted a regression analyses to examine different associations betwixt each individual infants' HF power and LF power. We chose changes in infants' HF power during the 0–5 min, v–ten min and 10–15 min periods as possible dependent variables and changes in mothers' and infants' LF power during the 0–five min, 5–x min and 10–15 min equally possible independent variables. The significance (p < 0.05) of the interaction term between each mean-centred independent variable and the moderator (developmental stage) was tested with each combination of contained-dependent variables. Data without technical bug from 22 mothers and 30 infants were subjected to analyses. Complete data from 19 dyads of mothers and infants were used for the ANOVA for TE and regression analyses. The basic attributes of the participants are presented in Table 4.
Data availability
All information analysed during this study are included in this published article (and its supplementary data files).
References
-
Shaffer, F., McCraty, R. & Zerr, C. L. A salubrious centre is non a metronome: an integrative review of the heart's anatomy and heart rate variability. Forepart. Psychol. 5, 1040 (2014).
-
DiPietro, J. A., Bornstein, M. H., Hahn, C. Due south., Costigan, K. & Achy‐Brou, A. Fetal heart rate and variability: stability and prediction to developmental outcomes in early babyhood. Kid Dev. 78, 1788–1798 (2007).
-
Field, T. & Diego, Thousand. Vagal activity, early on growth and emotional development. Infant Behav. Dev. 31, 361–373 (2008).
-
Van Leeuwen, P. et al. Is in that location evidence of fetal-maternal heart rate synchronization? BMC Physiol., three (2003).
-
Van Leeuwen, P. et al. Influence of paced maternal breathing on fetal–maternal heart rate coordination. Proc. Natl. Acad. Sci. 106, 13661–13666 (2009).
-
Van Puyvelde, M. et al. Whose clock makes yours tick? How maternal cardiorespiratory physiology influences newborns' centre rate variability. Biol. Psychol. 108, 132–141 (2015).
-
Bloch-Salisbury, E., Zuzarte, I., Indic, P., Bednarek, F. & Paydarfar, D. Kangaroo care: cardio-respiratory relationships between the infant and caregiver. Early on Hum. Dev. 90, 843–850 (2014).
-
Butruille, L. et al. Touch of skin-to-skin contact on the autonomic nervous organisation in the preterm infant and his mother. Baby Behav. Dev. 49, 83–86 (2017).
-
Feldman, R., Magori-Cohen, R., Galili, Thousand., Singer, M. & Louzoun, Y. Mother and infant coordinate heart rhythms through episodes of interaction synchrony. Infant Behav. Dev. 34, 569–577 (2011).
-
Izard, C. Eastward. et al. Infant cardiac activity: Developmental changes and relations with attachment. Dev. Psychol. 27, 432 (1991).
-
Bar-Haim, Y., Marshall, P. J. & Fox, N. A. Developmental changes in heart period and loftier-frequency heart period variability from 4 months to 4 years of age. Dev. Psychobiol. 37, 44–56 (2000).
-
Lehrer, P. M. et al. Centre rate variability biofeedback increases baroreflex gain and height expiratory menstruation. Psychosom. Med. 65, 796–805 (2003).
-
Vaschillo, E., Vaschillo, B. & Lehrer, P. Heartbeat synchronizes with respiratory rhythm only under specific circumstances. Chest 126, 1385–1386 (2004).
-
Dishman, R. K. et al. Heart rate variability, trait feet, and perceived stress among physically fit men and women. Int. J. Psychophysiol. 37, 121–133 (2000).
-
Ragnarsdottir, M. & Kristinsdottir, E. K. Breathing movements and breathing patterns among healthy men and women twenty–69 years of age - Reference values. Respiration 73, 48–54 (2006).
-
Lehrer, P. M. & Gevirtz, R. Heart charge per unit variability biofeedback: how and why does information technology work? Front. Psychol. 5, 756 (2014).
-
Marzbanrad, F., Kimura, Y., Endo, M., Palaniswami, Thousand. & Khandoker, A. H. Transfer entropy assay of maternal and fetal eye rate coupling. Conf. Proc. IEEE Eng. Med. Biol. Soc. 2015, 7865–7868 (2015).
-
Marzbanrad, F., Kimura, Y., Palaniswami, M. & Khandoker, A. H. Quantifying the interactions between maternal and fetal heart rates by transfer entropy. PLoS I ten, e0145672 (2015).
-
Karavidas, M. K. et al. Preliminary results of an open label report of middle rate variability biofeedback for the treatment of major low. Appl. Psychophysiol. Biofeedback 32, 19–30 (2007).
-
Steffen, P. R., Austin, T., DeBarros, A. & Chocolate-brown, T. The impact of resonance frequency animate on measures of heart rate variability, claret pressure, and mood. Frontiers in public health 5, 222 (2017).
-
Anishchenko, V. Due south., Balanov, A. G., Janson, North. B., Igosheva, Due north. B. & Bordyugov, Grand. 5. Entrainment between eye rate and weak noninvasive forcing. Int. J. Bifurcat. Chaos 10, 2339–2348 (2000).
-
Friston, K. The free-energy principle: a unified brain theory? Nat. Rev. Neurosci. xi, 127 (2010).
-
Seth, A. K. & Friston, Chiliad. J. Active interoceptive inference and the emotional brain. Philos. Trans. R. Soc. Lond. B Biol. Sci. 371, 20160007 (2016).
-
Akselrod, Due south. et al. Power spectrum analysis of centre-rate fluctuation - a quantitative probe of trounce-to-beat cardiovascular control. Science 213, 220–222 (1981).
-
Pomeranz, B. et al. Assessment of autonomic function in humans by eye rate spectral analysis. Am. J. Physiol. Centre Circ. Physiol. 248, H151–H153 (1985).
-
Badra, L. J. et al. Respiratory modulation of human autonomic rhythms. Am. J. Physiol. Heart Circ. Physiol. 280, H2674–H2688 (2001).
-
Sasano, N. et al. Straight issue of PaCO2 on respiratory sinus arrhythmia in conscious humans. Am. J. Physiol. Heart Circ. Physiol. 282, H973–H976 (2002).
-
Quintana, D. S., Alvares, Chiliad. A. & Heathers, J. A. Guidelines for reporting articles on psychiatry and heart rate variability (GRAPH): recommendations to accelerate research communication. Transl Psychiatry 6, e803 (2016).
Acknowledgements
The authors thank Dr. Takeshi Konno of Kanazawa Institute of Engineering for technical assistance on analyses of transfer entropy.
Author data
Affiliations
Contributions
A.S., M.U. & H.O. designed the study, analysed the data and drafted the paper, A.S. conducted the experiments and A.T. supported the data collection and measurement of biological indices. H.O. and H.I. supervised the project. All authors reviewed and agreed with the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Open up Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in whatever medium or format, every bit long every bit you requite appropriate credit to the original writer(s) and the source, provide a link to the Creative Eatables license, and indicate if changes were made. The images or other 3rd political party fabric in this article are included in the article's Creative Commons license, unless indicated otherwise in a credit line to the material. If material is non included in the article's Artistic Commons license and your intended utilize is not permitted by statutory regulation or exceeds the permitted use, you lot will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
Reprints and Permissions
About this article
Cite this article
Suga, A., Uraguchi, 1000., Tange, A. et al. Cardiac interaction between mother and infant: enhancement of middle rate variability. Sci Rep 9, 20019 (2019). https://doi.org/10.1038/s41598-019-56204-5
-
Received:
-
Accepted:
-
Published:
-
DOI : https://doi.org/ten.1038/s41598-019-56204-5
Comments
By submitting a comment you agree to abide by our Terms and Customs Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it every bit inappropriate.
Source: https://www.nature.com/articles/s41598-019-56204-5
0 Response to "Moms Heart Starts Beating Again From Baby"
Post a Comment